REDEEM Safety
Adverse events
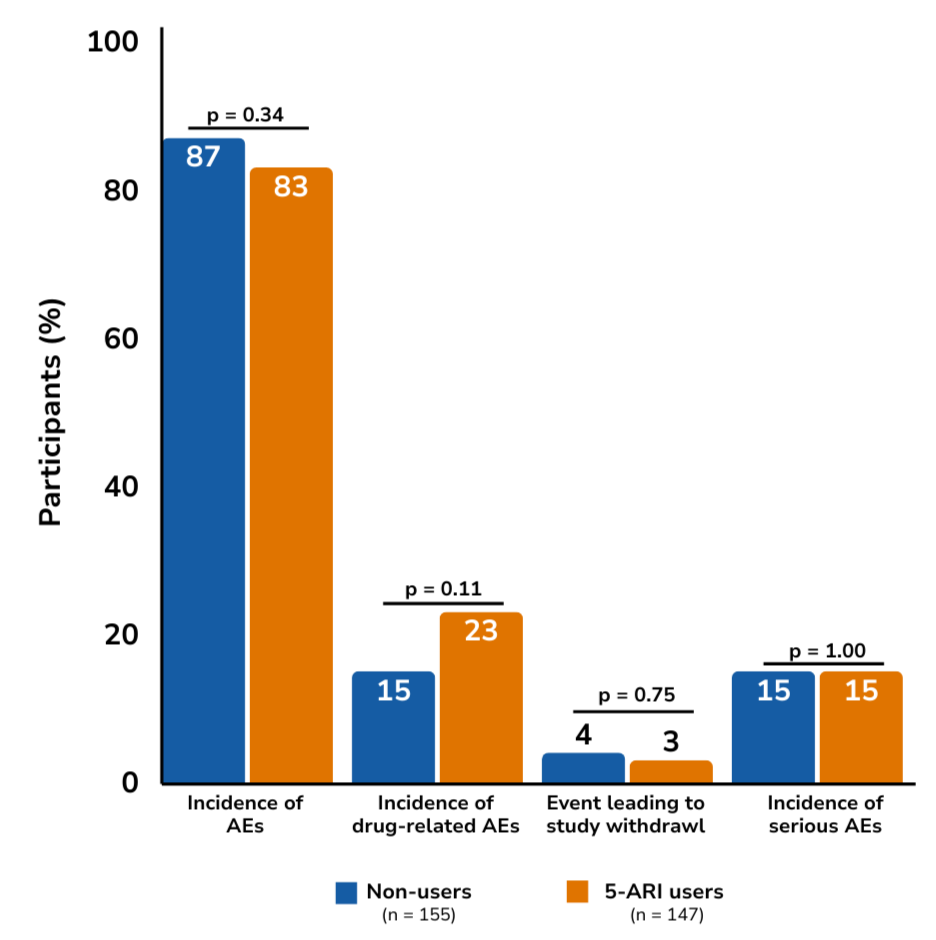
There were no significant differences in adverse events related to sexual function, breast disorders, or cardiovascular events. Sexual or breast-related adverse events occurred in 24% of men receiving dutasteride and 15% of men receiving placebo, and cardiovascular events occurred in 5% of men in both groups. No prostate cancer–related deaths or metastatic events were observed during the study period.