FOR U.S. HEALTHCARE PROFESSIONALS
Information on 5-Alpha Reductase Inhibitors (5-ARIs) During Active Surveillance For Prostate Cancer
Reboot Rx is a nonprofit focused on advancing affordable cancer treatments by repurposing generic drugs.
This site provides a consolidated overview of published studies and information on 5-ARIs, dutasteride and finasteride, in men with prostate cancer on active surveillance.
5-ARIs are not FDA-approved for patients with prostate cancer. This page is intended for informational purposes only and is not intended to provide medical advice and should not be relied upon in that regard. Clinicians should make individual prescribing decisions for their patients based on the patient's clinical presentation and the clinician’s own medical decision-making.
Across the studies summarized on this site, active surveillance populations primarily include men with low-risk (Gleason score 6, Grade Group 1) and favorable intermediate-risk (Gleason score 7 [3+4], Grade Group 2) prostate cancer. American Urological Association guidelines describe active surveillance and radical therapy as management options for these risk groups.1
Clinical Context
-
Active surveillance involves monitoring signs of disease progression over time and is often selected to defer treatments, such as prostatectomy or radiation, which may be associated with side effects.4,5 Population-based studies have reported increasing use of active surveillance in recent years.6
Active surveillance rates are increasing among patients with low-risk prostate cancer
-
Radical therapy options, such as prostatectomy and radiation, aim to remove or ablate prostate cancer tissue but have been associated with urinary, sexual, and bowel side effects in some patients.5,7 Reported risks vary across studies, techniques, and follow-up duration.
Some men initially managed with active surveillance later transition to radical therapy.2,3
5-ARIs
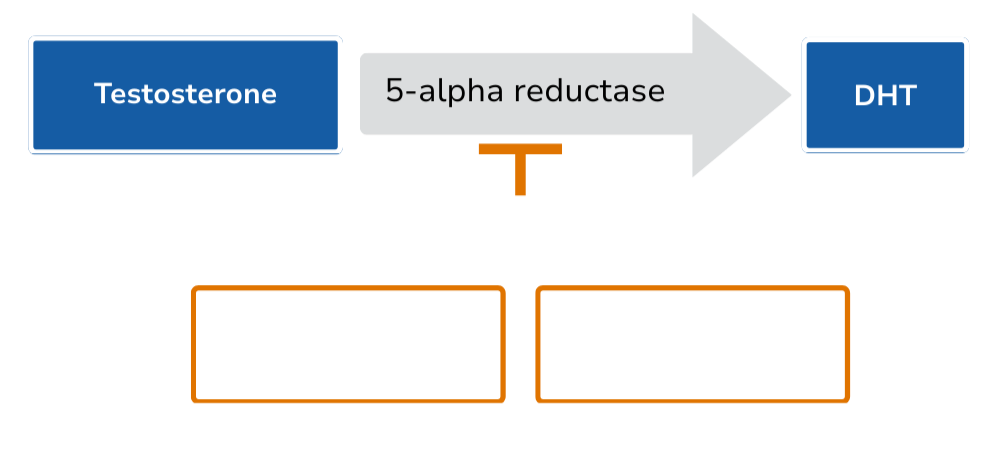
5-ARIs are oral generic drugs that reduce the conversion of testosterone to dihydrotestosterone (DHT).8 They are FDA-approved for the treatment of benign prostatic hyperplasia (dutasteride 0.5 mg; finasteride 5 mg) and androgenetic alopecia (finasteride 1 mg).9-11 5-ARIs have been evaluated for the purpose of delaying disease progression in multiple studies in men with prostate cancer on active surveillance.
Explore More:
Clinical History
1990s
5-ARIs first approved for the treatment of benign prostatic hyperplasia and androgenetic alopecia.9-11
2000s
Randomized controlled trials evaluated 5-ARIs in the context of prostate cancer prevention.12-13 These studies raised concerns regarding the effect of 5-ARIs on the detection of high-grade prostate cancer, which led to regulatory warnings and reduced clinical interest in this area.12-16
2010s
Later analyses suggested that observed differences in high-grade prostate cancer may have been influenced by a detection bias, with interpretation varying across studies.15,17-27
Randomized controlled trials, meta-analyses, and observational studies also evaluated 5-ARIs in men with prostate cancer on active surveillance.28-43
Learn more about our process for identifying generic drugs with repurposing potential.
References:
1. Eastham JA, et al. J Urol. 2022;208(1):10–8. 2. Hamdy FC, et al. N Engl J Med. 2023;388:1547–58. 3. Palmstedt E, et al. Eur Urol. 2025;88(4):373–80. 4. de Vos II, et al. J Pers Med. 2023;13(4):629. 5. Unger JM, et al. JAMA Oncol. 2024;10(12):1654–62. 6. Cooperberg MR, et al. JAMA. 2023;6(3):e231439. 7. CDC. Treatment of Prostate Cancer. Published February 11, 2025. Accessed January 6, 2026. https://www.cdc.gov/prostate-cancer/treatment/index.html 8. Chislett B, et al. Transl Androl Urol. 2023;12(3):487–96. 9. Finasteride (Proscar). Prescribing information. Merck & Co, Inc; 2021. Accessed November 6, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020180s047lbl.pdf 10. Dutasteride (Avodart). Prescribing information. GlaxoSmithKline; 2011. Accessed November 6, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021319s023s025lbl.pdf 11. Finasteride (Propecia). Prescribing information. Merck & Co, Inc; 2012. Accessed November 6, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020788s020s021s023lbl.pdf 12. Thompson IM, et al. N Engl J Med. 2003;349(3):215–24. 13. Andriole GL, et al. N Engl J Med. 2010;362(13):1192–202. 14. U.S. Food and Drug Administration. FDA Drug Safety Communication: 5-alpha reductase inhibitors (5-ARIs) may increase risk of more serious prostate cancer. Published June 9, 2011. Updated February 8, 2018. Accessed November 6, 2025. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-5-alpha-reductase-inhibitors-5-aris-may-increase-risk-more-serious 15. Theoret MR, et al. N Engl J Med. 2011;365(2):97–9. 16. Moul J. An interesting study during the evolution of chemoprevention. CancerNetwork. Published October 20, 2010. Accessed January 9, 2026. https://www.cancernetwork.com/view/primary-care-doctors-and-urologists-doubtful-about-using-finasteride-reducing-prostate-risk 17. Lucia MS, et al. J Natl Cancer Inst. 2007;99(18):1375–83. 18. Redman MW, et al. Cancer Prev Res (Phila). 2008;1(3):174–81. 19. Pinsky P, et al. Cancer Prev Res (Phila). 2008;1(3):182–6. 20. Chau CH, Figg WD. Nat Rev Urol. 2018;15(7):400–1. 21. Wallerstedt A, et al. J Natl Cancer Inst. 2018;110(11):1216–21. 22. Wang L, et al. Medicine (Baltimore). 2020;99(15):e19486. 23. Wilt TJ, et al. Cochrane Database Syst Rev. 2008;CD007091. 24. D’Amico AV, Barry MJ. J Urol. 2006;176(5):2010–12. 25. Thompson IM, et al. J Urol. 2007;177(5):1749–52. 26. Thompson IM, et al. J Natl Cancer Inst. 2006;98(16):1128–33. 27. Klotz L, et al. Can Urol Assoc J. 2012;6(2):83–8. 28. Fleshner NE, et al. Lancet. 2012;379(9821):1103–11. 29. Margel D, et al. J Urol. 2013;190(6):2039–45. 30. Moore CM, et al. J Urol. 2017;197(4):1006–13. 31. Finelli A, et al. Prostate Cancer Prostatic Dis. 2021;24(1):69–76. 32. Ashrafi AN, et al. World J Urol. 2021;39(9):3295–3307. 33. Kearns JT, et al. J Urol. 2019;201(1):106–11. 34. Özkan TA, et al. Turk J Urol. 2018;44(2):132–7. 35. Dai C, et al. J Urol. 2018;199(2):445–52. 36. Shelton PQ, et al. Urology. 2013;81(5):979–84. 37. Chiang AS, et al. Can Urol Assoc J. 2013;7(11–12):450–3. 38. Ross AE, et al. BJU Int. 2012;110(5):651–7. 39. Finelli A, et al. Eur Urol. 2011;59(4):509–14. 40. Barqawi AB, et al. Urology. 2010;76(5):1067–71. 41. Luo LM, et al. Asian J Androl. 2020;22(5):532–8. 42. Deng T, et al. PeerJ. 2020;8:e9282. 43. Matsukawa A, et al. Eur Urol Oncol. 2024;7(3):376–400.
Subscribe to receive updates on Reboot Rx and our work on 5-ARIs.
Reboot Rx is a registered 501(c)(3) nonprofit organization. Reboot Rx does not manufacture or sell drugs, and does not profit from the sale of drugs. Information provided on the Reboot Rx website (the “Information”), available at http://rebootrx.org (the “Site”), is intended for U.S. healthcare professionals only and not intended for patients. Information available on this Site is intended for informational purposes only and is not intended to provide medical advice and should not be relied upon in that regard. Reboot Rx provides this Information as an educational resource for the benefit of the medical community. The Information on this website has not been evaluated by the Food and Drug Administration. Reboot Rx is not a medical provider or health care facility and does not practice medicine. It thus can neither diagnose any disease or disorder, nor endorse or recommend any specific medical treatments. Nothing on this Site constitutes medical advice, recommendations as to the suitability of any specific product, service or information, or diagnoses for any medical condition. Any materials made available via the Site are provided for convenience and informational purposes only. Any clinician seeking to apply or consult the content and/or derivative sources on this website must use their independent medical judgment in the context of the individual clinical circumstances to determine any patient’s care or treatment.